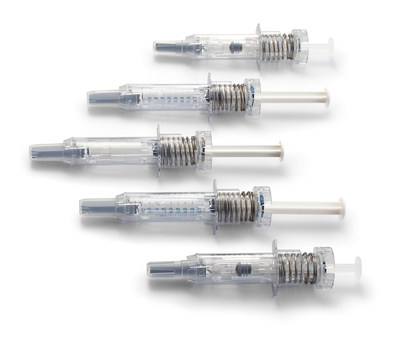
West's NovaGuard® SA Pro Safety System Recognized for Best Technologies Innovation at INTERPHEX
EXTON, Pa., Oct. 25, 2021 /PRNewswire/ -- West Pharmaceutical Services, Inc. (NYSE: WST), a global leader in innovative solutions for injectable drug administration, is pleased to announce that its NovaGuard® SA Pro safety system – a single-use accessory for prefilled ISO standard 0.5 mL and 1mL long staked-needle syringes – has won the INTERPHEX Best Technologies Innovation Award. The Best Technologies Innovation Award recognizes cutting-edge technologies, either new technologies or a novel implementation of existing technologies, that have the potential to change the way pharmaceutical and biotechnology companies operate.
"West is honored to receive this award for our NovaGuard SA Pro needle safety system, which is designed with innovative safety features to help eliminate accidental needlestick injuries," said Chris Ryan, Senior Vice President, Commercial Products and Emerging Markets at West. "We recognize that needlestick injuries are a serious hazard for healthcare practitioners, and we developed the NovaGuard system to provide greater control and enhanced safety when administering injections. West is proud of this continued innovation with our NovaGuard SA Pro safety device technology platform, and we continue to look for ways to deliver greater innovation in the marketplace to ultimately help our customers help their patients."
To help mitigate these needle stick injuries, the NovaGuard® SA Pro safety system has recently expanded its technology platform to include a 0.5mL device for standard 0.5mL glass syringes, in addition to the 1mL device that was already available for 1mL long glass syringes. This is important for injectable drug therapies such as low molecular weight heparin where drug manufacturers package and go to market with both 0.5mL standard glass syringes and 1 mL long glass syringes.
This safety system offers a low snap-in force during manufacturer assembly with the syringe, helps prevent pre-activation of the syringe, and provides a low activation for greater user comfort during administration. The NovaGuard SA Pro safety system also now has the C-clip integrated into the top portion of the design of the device enabling a one-step syringe assembly process for our customers in the biopharmaceutical industry.
West and the diamond logo and NovaGuard® are trademarks and registered trademarks of West Pharmaceutical Services, Inc., in the United States and other jurisdictions.
Forward-Looking Statements
Certain forward-looking statements are included in this press release. They use words such as "designed," "innovative," "eliminate," "provide," "enhanced," "help," "mitigate," "expanded," and other similar terminology. These statements reflect management's current expectations regarding future events and operating performance and speak only as of the date of this release. There is no certainty that actual results will be achieved in-line with current expectations. Specifically, there is no certainty that the NovaGuard® SA Pro safety device, either the newly offered 0.5mL device or the already available 1.0mL device, will achieve any particular result in the market. These forward-looking statements involve a number of risks and uncertainties. The following are some of the factors that could cause our actual results to differ materially from those expressed in or underlying our forward-looking statements: customer decisions to move forward with the product offering and dependence on third-party suppliers and partners. These important factors are not all inclusive. For a description of certain additional factors that could cause West's future results to differ from those expressed in any such forward-looking statements, see Item 1A, entitled "Risk Factors," in West's Annual Report on Form 10-K for the year ended December 31, 2020. Except as required by law or regulation, West undertakes no obligation to publicly update any forward-looking statements, whether as a result of new information, future events, or otherwise.
About West
West Pharmaceutical Services, Inc. is a leading provider of innovative, high-quality injectable solutions and services. As a trusted partner to established and emerging drug developers, West helps ensure the safe, effective containment and delivery of life-saving and life-enhancing medicines for patients. With almost 10,000 team members across 50 sites worldwide, West helps support our customers by delivering over 40 billion components and devices each year.
Headquartered in Exton, Pennsylvania, and in business for nearly a century, West in its fiscal year 2020 generated over $2.15 billion in sales. West is traded on the New York Stock Exchange (NYSE: WST) and is included on the Standard & Poor's 500 index. For more information, visit www.westpharma.com.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/wests-novaguard-sa-pro-safety-system-recognized-for-best-technologies-innovation-at-interphex-301407212.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/wests-novaguard-sa-pro-safety-system-recognized-for-best-technologies-innovation-at-interphex-301407212.html
SOURCE West Pharmaceutical Services, Inc.